Posted on by bee-health
Honey Bee Nutrition
CAP Updates: 10
Zachary Huang, Michigan State University
- Jointly published in the American Bee Journal and in Bee Culture, August 2010.
INTRODUCTION
Honey bees, like any other animal, require essential ingredients for survival and reproduction. What we know about honey bee nutrition now was learned mostly during the 50s-70s, and recent studies specifically on honey bee nutrition are very few. Honey bees require carbohydrates (sugars in nectar or honey), amino acids (protein from pollen), lipids (fatty acids, sterols), vitamins, minerals (salts), and water. Additionally, these nutrients must be present in the right ratio for honey bees to survive and thrive.
1. Carbohydrates
Like other animals, honey bees need carbohydrates as an energy source. All carbohydrates are first converted to glucose, which enters the Krebs cycle and produces ATP, the fuel in nearly all cells, and carbon dioxide and water as by-products. Aside from being used as an energy source, glucose can also be converted to body fats and stored. A worker bee needs 11 mg of dry sugar each day (Huang et al., 1998). This translates to about 22 ul of 50% sugar syrup per worker per day. A colony with 50,000 bees therefore needs 1.1 liter (about 2 pounds) of 50% sugar syrup per day (about half a gallon of nectar at 25% sugar concentration), which does not include brood rearing and other activities. A colony of this size, therefore will consume almost 700 pounds of nectar per year, assuming the nectars having a 50% sugar concentration,! Of course, consumption is lower during winter times when temperature is not regulated at 35C, but perhaps that cancels out the brood rearing and flight activities.
1.1. Collection of Nectar
Nectar is the main source of carbohydrates in the natural diet of honey bees. Sugar concentration in nectar can vary widely, from 5% to 75%, although most nectars are in the range of 25% to 40%. A honey bee uses her proboscis to suck up nectar from flowers and stores the liquid in her honey crop. The crop is a specialized part of the digestive system, and has a structure between it and the midgut, where digestion takes place. This structure, the proventriculus, can let some nectar in when the forager needs energy on its way home, remove pollen inside the nectar, and serve as a one-way valve to prevent backflow from the midgut. This ensures that no contamination of nectar or honey can take place. For this reason I tell people that honey is definitely not “bee vomit.” The honey crop is also the site of synthesis of ethyl oleate, a pheromone from foragers that tells young bees that they do not need to develop into foragers. The average weight of the nectar inside the crop is 25.5+15 mg (Calderone and Page, 1992), quite a feat considering that an average worker bee weighs 120 mg.
1.2. Conversion of Nectar into Honey
Foragers add enzymes (invertase, glucose oxidase) to nectar during foraging, so some digestion is already occurring before nectar is brought back to the hive. Invertase converts sucrose into two six-carbon sugars, glucose and fructose. A small amount of the glucose is attacked by the second enzyme, glucose oxidase, and gets converted into gluconic acid and hydrogen peroxide. Gluconic acid makes honey acidic, and hydrogen peroxide has germ-killing properties, both contributing to honey’s unfriendly disposition to bacteria, mold, and fungi. Foragers then pass the nectar to special “receiver” bees, which are middle-aged bees that have finished nursing, but have not started foraging yet. Receiver bees deposit nectar into cells and dry the nectar either on their mouthparts, by forming a large drop between the proboscis and the mandibles, or by fanning over the cells. The moisture has to be reduced to 17%-18% before bees consider the honey “ripe” and then seal the cells. Honey with high glucose levels (such as canola honey), will crystallize very quickly and should be extracted as soon as possible.
1.3. Toxic Substances in Nectar and Sugar Supplement
Adult bees can utilize glucose, fructose, sucrose, trehalose, maltose, and melezitose, but bees are unable to digest rhaminose, xylose, arabinose, galactose, mannose, lactose, raffinose, melibiose or stachyose. Most of these sugars are also toxic to honey bees. About 40% of sugars found in soybeans are toxic to bees, and therefore care should be taken when using soybeans as a pollen substitute.
Other plants are toxic to bees due to the presence of alkanoids in nectar. These include: azalea (Rhododendron molle), azure (Aconitum carmichaeli), black hellebore (Veratrum nigrum), California buckeye (Aesculus californica), Chinese alangium (Alangium chinense), Chinese bittersweet (Celastrus angulatus), jimson weed (Datura stramonium), plume poppy (Macleaya cordata), happy tree (Camptotheca acuminate), Summer Titi (Cyrilla racemiflora), tea (Camella sinensis) and oil-tea (C. olelfera). Nectar from these plants is usually toxic to both adult bees and brood, and the majority of them also toxic to humans.
Honey dews are sugary secretions produced by homopteran insects (aphids, leafhoppers, and woolly aphids). Honey dews are produced because the low protein diet (plant sap) that these insects rely on force them to drink excess fluids to obtain enough amino acids, and thus need to secrete the excess sugary water. Honey bees will collect honey dews to make honey dew honey. This type of honey is praised by some people due to its strong and unique flavor, but can cause dysentery in overwintering bees due to indigestible sugars or high levels of minerals. Adult bee paralysis in bees in Germany was also attributed to high Potassium and/or Phosphorus and low Sodium concentrations.
HMF (hydroxymethylfurfural) is formed in honey and high fructose corn syrup (HFCS) at high temperatures due to acid-catalyzed dehydration of hexose sugars, with fructose more prone to its formation. HMF above 30 ppm (parts per million) is considered toxic to honey bees. HFCS with such levels of HMF has been found to cause high mortality in cage studies (LeBlanc et al., 2010), as well as higher mortality than bees infected with Nosema ceranae (Z.Y. Huang, unpublished data). Beekeepers using HFCS for bee feeding should pay special attention to storage conditions, although many times, the batch from the supplier might have already become “bad” due to high temperatures either during transportation or storage.
Some honeys are not toxic to bees, but to humans. A good example is honey from tutu (Coriaria arborea), which has caused fatalities in New Zealand.
2. Protein
2.1. Importance of Pollen
Pollen provides bees with protein, minerals, lipids, and vitamins (Herbert and Shimanuki, 1978). All animals need essential amino acids, which must be obtained externally and cannot be synthesized by animals. Honey bees also need the same 10 amino acids (see section 2.5) as other animals (e.g., humans). These amino acids are obtained from pollen only, because honey bees do not have any other sources of protein. Pollen collection by a colony ranges from 10-26 kg per year (Wille et al., 1985). When honey bees are provided with insufficient pollen, or pollen with low nutritional value, brood rearing decreases (Turner et al., 1973; Kleinschmidt and Kondos, 1976, 1977) and workers live shorter lives (Knox et al., 1971). These effects ultimately affect colony productivity (reviewed by Keller et al., 2005). Shortages of pollen during rainy seasons can cause colony decline or collapse (Neupane and Thapa, 2005). Recent studies have shown that spring pollen supplement can work as insurance (when spring weather is bad) for faster spring buildup and higher honey yield (Mattila and Otis, 2006a), and can reduce the effects of varroa parasitism (Janmaat and Winston, 2000) and nosema infection (Mattila and Otis, 2006b).
2.2. Collection of Pollen
Pollen is collected either by pollen foragers, which specialize on pollen collection, or nectar-foragers, which happen to be dusted with pollen. Pollen is brushed off the worker’s body by the front and middle legs, and transferred to a special structure in the hind leg called the cubicula, or pollen basket. Pollen foragers unload their pollen by “kicking” the pollen pellets off their legs into a cell, which often already has pollen in it, and then the pollen pellets are “hammered” into a paste-like consistency by other workers. Due to the secretions added by bees, the pollens in each cell go through a lactic fermentation. The main effects of fermentation seem to be the reduction of starch (from 2% to 0%), increases in both reducing sugars and fiber, and reduction of ash and pH (Herbert and Shimanuki, 1978). Three bacteria that might contribute to lactic acid fermentation are found in bee bread: Pseudomonas, Lactobacillus, and Saccharomyces. Recently, it is shown that pollen collected by bees can easily be inoculated and fermented, and bees consumed it in the same way they consume unfermented pollen (Ellis and Hayes, 2009).
The weight of two pollen pellets from a pollen forager ranges from 7.7-8.6 mg (Rose et al., 2007). A colony will collect more pollen if it has more brood pheromone, more queen pheromone, or is genetically disposed to collect more pollen. Robert Page (currently at Arizona State University) has selected high and low pollen hoarding lines, whereby the high pollen line will collect so much pollen that there is no room to rear brood, and the low pollen line will perish without supplementing pollen artificially.
2.3. Processing Pollen into Proteins
Pollen is mixed with glandular secretions to produce “bee bread,” which is consumed by young bees, considered the “social stomach” for protein digestion (because foragers cannot digest pollen directly, but still need protein (Moritz and Creilsheim, 1987). Rearing one larva requires 25-37.5 mg protein, equivalent to 125-187.5 mg pollen (Hrassnigg and Crailsheim, 2005). Newly emerged bees have undeveloped hypopharyngeal and mandibular glands. Hypopharyngeal glands are paired glands inside worker’s head, consisting of a long central duct with many “grapes” (acini) attached. The glands will only develop after consuming a lot of pollen for the first 7-10 days. The glands first secrete the protein-rich component of royal jelly in young bees, but then secrete invertase, which is used to convert sucrose to simple sugars (fructose and glucose), in foragers. Mandibular glands are simple, sac-like structures attached to the base of each mandible. The glands secrete lipid-rich components of the royal jelly in young bees, but produce an alarm pheromone (2-heptanone) in foragers.
2.4 Royal Jelly Composition
Royal jelly (RJ) is 67% water and 32% dry matter. The dry matter is composed of 12.1% carbohydrates, 4.0% lipids, 12.9% proteins, and 1.1% ash (Wangchai and Ratanavalacai, 2002). These percentages vary slightly in different seasons. RJ also contains many trace minerals, some enzymes, antibacterial and antibiotic components, and trace amounts of vitamin C. The fat-soluble vitamins, A, D, E and K, are absent from royal jelly. The 13% of total proteins consists of 52 different proteins (Yu et al., 2009). The majority of the identified proteins (47 out of 52) are major royal jelly proteins (MRJPs), named as MRJP1 through 6, each of which has many variations. Three enzymes were also detected in the RJ: glucose oxidase, peroxiredoxin, and glutathione S-transferase.
It is no doubt that RJ is highly nutritious for bee larvae. Bee larvae grow exponentially during their first 4.5 days of life, from 0.36 + 0.008 mg (12 hr larvae) to 131.44±18.7 mg (4.5 days), reaching a weight of 159.66±12.91 mg after being capped (Petz et al., 2004). The weight gain is nearly 1000 times when compared to the weight of the eggs (0.17 mg, Taber et al., 1963). Furthermore, bee larvae do not defecate at all during the first 5 days of life, which is necessary because otherwise larvae would be feeding on their own waste. The midgut and hindgut are not connected until the last molt into the mature larvae, therefore preventing the possibility of defecation. After defecation, the larva stops feeding, starts spinning a cocoon, and straightens itself along the cell axis, and becomes a prepupae. Three days later it will pupate and eventually, (after one week) emerge as an adult. It is not yet clear what role(s) the major royal jelly proteins play in honey bee larvae nutrition. Larvae can survive on an artificial medium without RJ or proteins for 3-4 days, but they all die 1-2 days before defecation (Z.Y. Huang, unpublished results). Until a chemically defined media is available for honey bee larvae, we will not know the roles various components of RJ play in larval growth and development.
2.5. Measurements of Pollen Quality
Pollen quality can be measured by two methods: crude protein levels or the composition of amino acids. Ten amino acids have been found to be “essential” for honey bees (deGroot, 1953), meaning that bees cannot synthesize or even convert other amino acids to acquire them, and therefore must obtain them directly from food, either as free amino acids or digested from protein. These 10 amino acids are listed in Fig. 1.
The crude protein level tells us how much protein a particular plant pollen has, and higher crude protein levels are better than lower ones. However, if the 10 amino acids are not balanced, bees cannot fully use what is available in the pollen. For example, Fig. 1 shows that honey bees need 4% isoleucine from the total available amino acids, if one type of pollen has only 2% isoleucine, then bees can only use 50% of the total protein because isoleucine will be the limiting factor (Stace, 1996), forcing bees to ingest twice the amount of total pollen to obtain the needed isoleucine, essentially wasting half of the total protein.
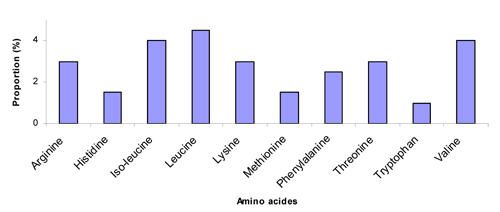 Fig. 1. Proportion (%) of the 10 essential amino acids needed by honey bees (deGroot, 1953).
Fig. 1. Proportion (%) of the 10 essential amino acids needed by honey bees (deGroot, 1953).
2.6. Not All Pollens Are Created Equal
Different pollens have different nutritional value to honey bees. Schmidt et al. (1987) studied the nutritional value of 25 pure pollens by feeding caged bees the different pollens, using sugar as a negative control, and mixed pollen as a positive control. Consumption of test pollen diets varied dramatically among test pollens, with a mean consumption of 16.5 mg pollen per bee for the first 10 days and a range of 1.9-29.0 mg per bee. Both pollen consumption rates and crude protein levels are correlated with the ability to improve longevity. Pollens that decreased worker longevity include ragweed (Ambrosia), a rust spore (Uromyces), cattail (Typha), and Mexican poppy (Kallstroemia). Those that slightly improved worker longevity include terpentine bush (Haplopappus), desert broom (Baccharis), and dandelion (Taraxacum). The best pollens are those from Mormon tea (Ephedra), mesquite (Prosopis), blackberry (Rubus), and cottonwood (Populus) Mixed pollen consistently performed very well. In another study, Schmidt et al. (1995) concluded that bees foraging in sesame and sunflower fields should be supplemented with other pollen, but rapeseed (canola) pollen is highly nutritious to bees and does not need supplementing. Through these studies, Schmidt concluded that factors contributing to increased bee longevity include presence of attractants and phagostimulants, so that bees will readily consume large amounts of pollen; lack of toxic compounds; and a good nutrient balance or level. No studies have tried to correlate the amino acid profile of a pollen and its ability to improve worker longevity.
A few pollens are toxic to honey bees, with some killing the adults (e.g., Zigadenus), others killing the brood (e.g., Heliconia). Other plants with toxic pollen are balsa (Ochroma lagopus), California buckeye (Aesculus californica), and Flame of the Forest (Spathodea campanulata).
2.7. Pollen Substitute for Bees
A good pollen substitute for honey bees should have the same features as a good pollen: 1). palatability (bees will readily consume it), 2). Digestibility (it is easily digested by bees), and 3). Balance (it has the correct the amino acid balance and enough crude proteins). Currently there are four commercial pollen substitutes for honey bees in the U.S.: Bee-Pol®, Bee-Pro®, Feed-Bee®, and MegaBee®. It appears that Bee-Pro® is soy-based, and Feed-Bee® and MegaBee are non-soy-based. I have insufficient information for Bee-Pol.
Cremonez et al. (1998) fed caged bees various diets and used hemolymph protein titer to assess their quality, with higher protein titer suggesting higher quality. Six day old bees had protein concentration of 27.6, 24.1, 11.4, 3.98, and 2.2 ug/ul, for bee bread, soybean/yeast, pollen, corn meal and sucrose, respectively. De Jong et al. (2009) used the same assay to assess the quality of commercial pollen substitutes. They found that bees feeding on Feed-Bee®, Bee-Pro®, pollen, acacia pod flour diets and sucrose had hemolymph titers of 9.42, 8.95, 6.26, 6.0 and 3.56 ug/ul, respectively. It would be informative to see if the high protein in blood translates to longer life in either cages or small colonies.
Gregory (2006) reported that for longevity inside small colonies of bees fed different diets, ranked by superiority: fresh pollen > Feed-Bee® > Bee-Pro® > old pollen. In cage studies, Feed-Bee® had similar hemolymph protein to fresh pollen. She also reported that Feed-Bee® contained 34.9 mg sucrose and 2.03 mg stachyose, while Bee-Pro® contained 8.85 mg sucrose and 4.55 mg stachyose. Stachyose is toxic to honey bees unless it is diluted to below 4% with 50% sucrose.
Degrandi-Hoffman et al. (2008) evaluated three diets, Bee-Pro®, Feed-Bee®, and MegaBee®, in two separate trials. In both trials, Bee-Pro® and MegaBee® patties were consumed at rates similar to pollen cake, but Feed-Bee® was consumed significantly less. Higher food consumption was significantly correlated with increase in brood area and adult population size. According to this study, MegaBee appeared to be superior to both Bee-Pro® and Feed-Bee® in terms of brood production or adult population.
2.8. Pollen Nutrition May Play a Role in CCD
Recently, a new threat, Colony Collapse Disorder (CCD), emerged to attack the honey bees in the U.S. and has caused 30%-40% loss of bee colonies each year since the fall of 2006 (CCD working group, 2007). CCD-affected colonies have greatly reduced adult bee populations, with only a few hundred workers and the queen left, but with many frames of brood, which suggests rapid depopulation of adults. The cause of CCD remains unknown, but many scientists believe that it may be caused by a combination of factors, such as pesticides, parasites, nutritional stress, and stress from long distance transportation. There is a growing body of evidence showing that poor nutrition can be a major player in affecting honey bee health. Eischen and Graham (2008) demonstrated that well-nourished honey bees are less susceptible to Nosema ceranae than poorly nourished bees. Honey bees that were treated with imidacloprid and fed Nosema spp. spores suffered reduced longevity and reduced glucose oxidase activity, indicating an interaction between the two factors (Alaux et al., 2010a). Naug (2009) tested the hypothesis that nutritional stress due to habitat loss has played a major role in causing CCD by analyzing the land use data in U.S. He showed a significant correlation between the number of colony loss due to CCD from each state and the state’s ratio of open land relative to its developed land area. Furthermore, Naug showed that these states with the largest areas of open land have significantly higher honey production. It therefore appears that honey plants (especially those in natural, undeveloped areas) might play a major role in honey bee health.
2.9. Polyfloral Diets Healthier for Honey Bees
Schmidt conducted a series of studies and convincingly showed that in general, mixed pollen given to caged bees let bees live longer than those on a single species of pollen (Schmidt, 1984; Schmidt et al., 1987, 1995). In a very recent study, Alaux et al. (2010b) showed that polyfloral diets from mixed pollen enhanced some immune functions compared with monofloral diets, in particular glucose oxidase activity, suggesting that the diversity in floral resources provided bees with better in-hive antiseptic protection. These studies suggest that bees feeding on a single type of pollen are not as healthy as those on a variety of pollens. With the modern way of agriculture— increasingly larger areas of mono-cultured crops— honey bee health might be adversely affected.
3. Other Nutrition
3.1. Sterols and Lipids
A sterol, 24-methylene cholesterol, is common in pollen and is the major sterol source for honey bees. Nearly all insects need to obtain sterol from their diet because of their inability to synthesize them directly. Sterol is the precursor for important hormones such as molting hormone, which regulates growth because it is required at the time of each molt. It is not clear what other lipids are required by honey bees, but most likely normal consumption of pollen provides for all the lipid requirements. Pollen with low fat content is less likely to be consumed by honey bees, but can be made more attractive to bees with the addition of lipids. The total lipid concentration within a pollen supplement is recommended to be 5%–8%.
3.2. Vitamins
Nurse bees are thought to need the following vitamin B complex for brood rearing: thiamine, riboflavin, nicotinamide, pyridoxine, pantothenic acid, folic acid, and biotin. Ascorbic acid (vitamin C) also seems essential for brood rearing. Like sterol and lipids, the vitamin needs of a honey bee colony are satisfied if pollen stores are abundant in the hive or fresh pollen is being brought into the colony. It is not known whether micro-organisms naturally present in the alimentary canal of bees may play a role in providing vitamins and other essential substances.
3.3. Minerals
The mineral requirements of honey bees are poorly understood. High amounts of potassium, phosphate, and magnesium are required by all other insects, and so presumably are by honey bees as well. Excessive levels of sodium, sodium chloride, and calcium have been shown to be toxic to honey bees. Again, all the required minerals can be obtained from pollen, although nectar also contains minerals. Dark honey contains higher levels of minerals. The optimal ash concentration for maximum brood rearing seems to be at 0.5%–1%. Pollen with more than 2% ash inhibits brood production.
3.4. Water
Honey bees forage for water for two purposes. One is to use it to dilute honey so that honey can be added to brood food. The second is to use water to cause evaporative cooling by fanning over a thin layer of water when the ambient temperature is over 35° C. During winter time, bees have enough water from condensation over the inner cover, so the issue is usually too much water, which can drip on the cluster and kill bees if there is not adequate ventilation. When bees have a choice, they usually prefer water with some salts (e.g. a swimming pool over a lake). Other species of honey bees (e.g. Apis dorsata, A. cerana) have been observed to forage on urinals or open restrooms in Asia. This is probably because bees are not obtaining adequate sodium from their nectar or pollen.
CONCLUSIONS
Honey bees can obtain all of their nutrients naturally if bees are in a natural setting. Unfortunately, modern agriculture has necessitated large scale mono-cropping which can be harmful to honey bees. This is mainly because each plant species has a specific nectar or pollen characteristic. Much like humans, a lack of variety in foods can cause problems. Many studies have shown poly-floral pollen diets are superior to a single species of pollen, with perhaps one exception (rape seed pollen alone can be excellent). We urgently need to understand the implication of each mono-culture crop on honey bees. For example, how much stress do bees experience when feeding exclusively on almond nectar and pollen for 3-4 weeks? How long do they need to (or can they?) recover after the stressful period? Are there “supplemental” crops available to reduce or eliminate such a stress? By understanding these questions and providing solutions to them, we will be able to make bees as healthy as possible.
ACKNOWLEDGEMENT
I thank Xishuangabanna Tropical Botanical Garden and Shangri-La Alpine Botanic Garden for hosting my stay in China while this paper was written (June 13-19, 2010). I also thank Melissa Huang and Katrina Klett for reviewing the paper.
FURTHER READING
- Brodschneider, R., K. Crailsheim. 2010. Nutrition and health in honey bees. Apidologie DOI: 10.1051/apido/2010012
- De Groot, A.P. 1953. Protein and amino acid requirements of the honeybee Apis mellifera. Physiologia Comparata et d’Ecogia. 3: 195-285.
- Schmidt J.O., S.C. Thoenes, M.D. Levin. 1987. Survival of honey bees, Apis mellifera (Hymenoptera: Apidae), fed various pollen sources, Ann Entomol. Soc. Am. 80: 176–183.
- Somerville, D. 2005. FAT BEES SKINNY BEES – a manual on honey bee nutrition for beekeepers. NSW Department of Primary Industries. RIRDC Publication No 05/054. https://rirdc.infoservices.com.au/downloads/05-054.pdf
Originally published in 2010 at beehealth Extension.org. Moved here Feb 21, 2021.
[testing editing Nov 10, 2021]


Hi! My name is Rita Robinson, a beekeeper, currently taking an environmental science class at Ohio State, Marion Campus. The focus of my research poster is fragmentation of forage and honey bee decline in Ohio. So, 1st, I’m older and not very computer savvy so go easy on me if you answer this. 2nd, I’d like to use your article and cite it for my poster, if that’s ok. I promise not to plagiarize. Your summation of nutritional needs is terrific. And, lastly, can you recommend any scientific articles written about this topic, anywhere in the U.S.? I wanted to look at effects in Central Ohio but I’m not finding related articles and most of those I do find are singularly disease/pesticide focused, and rightly so. But isn’t anybody worried about our bees having only food contaminated with chemicals from crops and crop drift?
Of course it will be ok to cite any of papers/writings. Please google my name for my email and we can discuss further.